
BioCleanse for BTB allografts in ACL rupture performs comparably to the aseptic technique

BioCleanse for BTB allografts in ACL rupture performs comparably to the aseptic technique
Aseptically processed and chemically sterilized BTB allografts for anterior cruciate ligament reconstruction: a prospective randomized study
Knee Surg Sports Traumatol Arthrosc. 2013 Sep;21(9):2107-12Did you know you're eligible to earn 0.5 CME credits for reading this report? Click Here
OE EXCLUSIVE
Dr. Peter Indelicato discusses the effects of irradiation on allografts tissue for ACL reconstruction and develops the rationale for the BioCleanse study presented in this ACE Report.
Synopsis
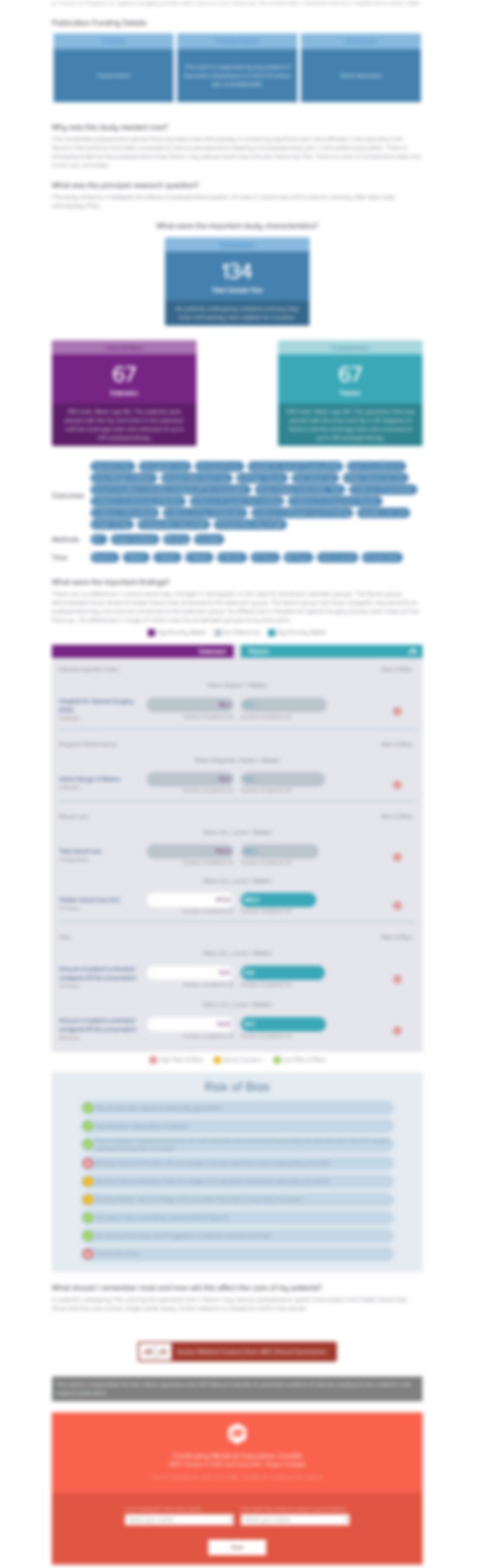
67 patients with an acute (<4 months), isolated, unilateral ACL rupture were randomized to evaluate the efficacy of the BioCleanse sterilization technique against traditional aseptic processing for bone-patellar tendon-bone (BTB) allografts. Patients were assessed over 24 months using the International Knee Documentation Committee (IKDC) form outcome, range of motion (ROM) effusion and a KT-1000 k...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue


 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.