
No increase in adverse events with liposomal bupivacaine vs placebo in elective lumbar spine surgery

No increase in adverse events with liposomal bupivacaine vs placebo in elective lumbar spine surgery
Can Liposomal Bupivacaine Be Safely Utilized in Elective Spine Surgery?
Global Spine J. 2019 Apr; 9(2): 133–137.Did you know you're eligible to earn 0.5 CME credits for reading this report? Click Here
Synopsis
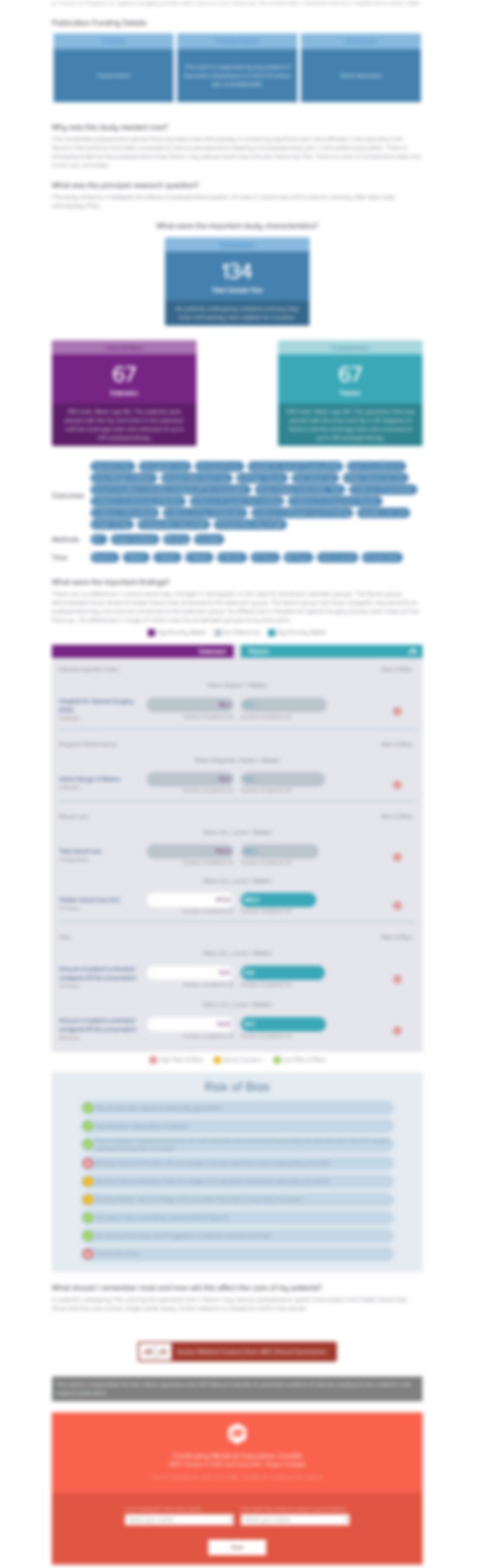
59 patients with degenerative spondylosis scheduled for elective open posterior lumbar decompression and instrumented fusion were randomized to receive a pre-wound closure injection of liposomal bupivacaine (266mg) or placebo for the treatment of post-operative pain. The primary outcome of interest was the incidence of adverse events. The secondary outcome of interest was the length of hospital st...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue


 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.